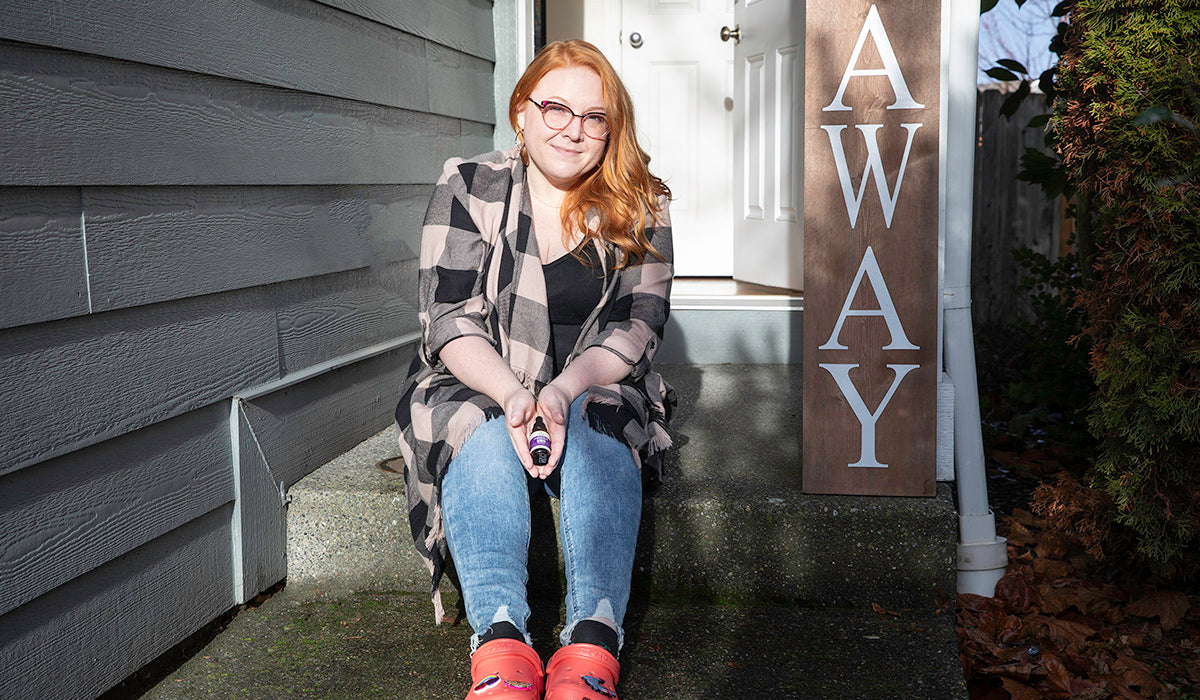CBD FOR ALL | CUSTOMER STORY: JESSICA S.
Living on the western slopes of Mt. Rainier in Puyallup, Washington has meant many long, dark, and rainy winters for Jessica Shook. Those months of low clouds and rare sunshine used to be especially challenging for her. Over a decade ago, Shook found herself at her doctor’s office, hoping to get answers for the heart-pounding anxiety and frequent tears she couldn’t explain. She was prescribed standard pharmaceutical anxiety treatment, but after trying a couple of different brands she didn’t feel all that much better. “Pharmaceuticals I think give me more anxiety… their side effects were awful, I just didn’t want...
Our Process | Formulation and Packaging
Formulation and packaging culminates months of work from when we first put our hemp seeds into soil. At this point, our hemp has been grown, harvested, extracted, and distilled. Our team in Seattle has created barrels of potent full spectrum hemp oil, CBD distillate, and CBD (or CBG isolate). These cannabinoid-rich concentrations made by our extraction team are shipped south to Portland for the exciting final stage of our internal process - making and packaging our products. Building the right recipe The trick to making great tasting, effective products starts in our test kitchen. Whether it’s finding the balance of...
Our Process | Extraction and Distillation
After weeks of harvesting, our drying barn is filled with thousands of boxes of cured hemp flower. It will take months to process this dense, potent biomass. Batch by batch, we truck the hemp over to our state-of-the-art extraction lab, just a few hundred feet away. The process of extracting the cannabinoids necessary to ultimately craft our entire suite of CBD products requires a bit of a science lesson. Much like the extraction process itself, we’re here to break it down for you. How extraction works The first step is to transfer the hemp flower into large mesh bags —...
Our Process | Growing and Harvesting Hemp
From the time our plants go into the ground in April and May until they’re harvested, there’s a lot of work to be done. We focus our efforts on growing hemp as efficiently, sustainably, and consistently as possible — which is no small feat given some of the variables mother nature throws our way.
Recent articles